Abstract:
The present study reports the stability constants
of complexes with fatty acids: Alpha Linolenic Acid (ALNA),
Arachidonic Acid (AA) and Oleic acid (OA) with Co2+,
Ni2+, Cu2+, Zn2+, Cd2+,
Sn2+, Hg2+ and Pb2+ ions in
water-ethanol medium 50% v/v at three different temperatures
25ºC, 35ºC and 45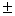 0.1ºC
keeping ionic strength constant (0.1 M KNO3).
Experiments were conducted in 1000 mL jacketed glass reaction
vessel under nitrogen atmosphere. Hydrogen ion concentrations
were measured and the stability constants were calculated from
the potentiometric titration curves. The order of the stability
constants of the formed complexes decreases in the sequence Cu2+,
Cd2+, Co2+, Ni2+, Pb2+,
Zn2+, Hg2+
0.1ºC
keeping ionic strength constant (0.1 M KNO3).
Experiments were conducted in 1000 mL jacketed glass reaction
vessel under nitrogen atmosphere. Hydrogen ion concentrations
were measured and the stability constants were calculated from
the potentiometric titration curves. The order of the stability
constants of the formed complexes decreases in the sequence Cu2+,
Cd2+, Co2+, Ni2+, Pb2+,
Zn2+, Hg2+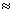 Sn2+.
The effect of temperature was also studied and the corresponding
thermodynamic functions (ΔG, ΔH and ΔS) were derived and
discussed. The formation of metal complexes has been found to be
spontaneous and entropically favourable. The method of Calvin
and Bjerrum as adopted by Irving and Rossotti has been employed
to determine log K1 values. This order is in good
agreement with Irving - Williams order of stability.
Sn2+.
The effect of temperature was also studied and the corresponding
thermodynamic functions (ΔG, ΔH and ΔS) were derived and
discussed. The formation of metal complexes has been found to be
spontaneous and entropically favourable. The method of Calvin
and Bjerrum as adopted by Irving and Rossotti has been employed
to determine log K1 values. This order is in good
agreement with Irving - Williams order of stability.
KEYWORDS: Stability
constant; Fatty acids; Potentiometric titration; Thermodynamic
functions.

